Press release
PROTECT III Presented at TCT 2019 – Clinical Data Demonstrates Protected PCI with Impella is Associated with Improved Outcomes
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190926005305/en/
PROTECT III Presented at TCT 2019 – Clinical Data Demonstrates Protected PCI with Impella is Associated with Improved Outcomes
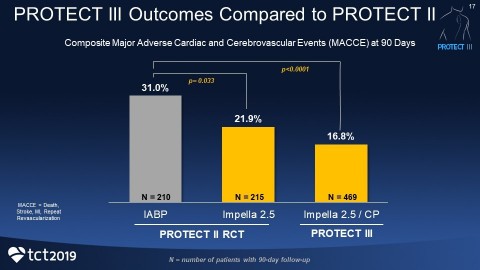
PROTECT III demonstrates a reduction in the primary endpoint of death, stroke, myocardial infarction and repeat procedures at 90 days with Impella-supported Protected PCI, compared to PROTECT II. Death, stroke, myocardial infarction and repeat procedures are known collectively as major adverse cardiac and cerebrovascular events (MACCE).
The findings of this interim analysis on 898 patients were announced today at the 31st Transcatheter Cardiovascular Therapeutics (TCT) conference by a member of the study’s steering committee,
The PROTECT series of
Protect III Findings Announced
Patients in PROTECT III are statistically older (71 years), include more women (26%) and non-Caucasians (33%), received longer support and had more complex procedures with more vessels treated than patients in PROTECT II. Yet the 90-day MACCE rate in PROTECT III is lower than the intra-aortic balloon pump (IABP) control arm from PROTECT II. The composite MACCE rate in the IABP arm was 31%, compared to the Impella 2.5 and Impella CP arm at 16.8% (p<0.0001).
“Based on the learnings from the PROTECT studies, decisions to provide hemodynamic support during PCI should be made in the context of providing complete revascularization and patient outcomes should include in-hospital and out-of-hospital improvements,” said Dr. Popma. “Prior studies have demonstrated it is important to achieve complete revascularization because it can result in a 30-50% reduction in MACCE, compared to incomplete revascularization.”
Summary of Acute Kidney Injury (AKI) Data
High-risk PCI patients face elevated risk of AKI due to high levels of contrast and long procedures. Additionally, chronic kidney disease is a common co-morbidity. According to a NCDR Cath PCI Registry Study of 985,737 patients, in-hospital mortality for patients with AKI is 10% and escalates to 34% if dialysis is required.
In PROTECT III, a sub-study (N=106) of patients were evaluated for acute kidney injury (AKI) rate and compared to a propensity matched control group (N=106). The results will be presented during the TCT Scientific Sessions. Multiple studies with Impella have measured the AKI rate based on predicted AKI risk factors, including contrast volume. These include:
- Circulation, 2012 -The PROTECT II RCT found Impella patients had statistically higher contrast volume than the IABP arm (267 ml vs. 241 ml, p=0.04), but a numerically lower rate of acute renal dysfunction at 30 days and 90 days (p=0.792 and p=0.776, respectively), showing a decoupling of contrast volume from renal dysfunction
-
Circulation Research , 2017 - Flaherty, et al., identified a six-fold reduction in AKI requiring dialysis when Impella support was used, compared to without Impella support (p=0.031). - Catherization and Cardiovascular Interventions, 2019 – Flaherty et al., found, compared to a predicted rate of 22% (using the Mehran risk score), only 5% of Impella-supported patients developed AKI (exclusively stage 1) at 48 hours, representing a 77.6% lower AKI risk (p<0.0001).
The PROTECT Series
The PROTECT III Study includes 898 patients enrolled at 45 sites in
“The totality of clinical data in favor of Impella supported high-risk PCI allows interventional cardiologists to be confident they are using the optimal treatment and technologies to help achieve complete revascularization in a single setting, improve procedural hemodynamic stability and improve patient quality of life,” said William O’Neill, MD, medical director of the
The
A video of Dr. Popma commenting on the results of PROTECT III and slides from Dr. Popma’s presentation at TCT are available online at www.ProtectIIIStudy.com. Dr. Popma’s presentation from TCT will be posted at www.ProtectIIIStudy.com within the next 24 hours.
The study is sponsored by
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5™ with Smart Assist® are U.S.
In
To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT
Based in
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of